|
Beryllium atom structure
Wave theory-United nature theory
This work is based on nature and laboratory
observations.
Tejman Chaim Henry Dr.
Name: Beryllium
Symbol: Be
Atomic Number: 4
Atomic Mass: 9.012182
Melting Point: 1278.0 °C (1551.15 K, 2332.4 °F)
Boiling Point: 2970.0 °C (3243.15 K, 5378.0 °F)
Number of Protons/Electrons: 4
Number of Neutrons: 5
Classification: Alkaline Earth
Crystal Structure: Hexagonal
Density @ 293 K: 1.8477 g/cm3
Color: gray
Atomic Structure
|
![[Bohr Model of Beryllium]](Beryllium_atom_structure_files/image001.gif)
|
|
Number of Energy Levels: 2
First Energy Level: 2
Second Energy Level: 2
|
|
Isotope
|
Half Life
|
|
|
Be-7
|
53.3 days
|
|
|
Be-9
|
Stable
|
|
|
Be-10
|
2600000.0 years
|
|
|
|
|
|
|
|
Date of Discovery: 1798
Discoverer: Fredrich Wohler
Name Origin: From the mineral beryl
Uses: spacecraft, missiles, aircraft
Obtained From: beryl, chrysoberyl
http://environmentalchemistry.com/
·
Atomic
Radius: 1.4Å
·
Atomic
Volume: 5cm3/mol
·
Covalent
Radius: 0.9Å
·
Cross
Section (Thermal Neutron Capture) a/barns: 0.0092 a/barns: 0.0092
·
Crystal
Structure: Hexagonal

·
Electron
Configuration:
1s2 2s2
·
Electrons per Energy
Level: 2,2
Shell Model
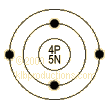
·
Ionic
Radius: 0.35Å
·
Filling
Orbital: 2s2
·
Number of Electrons
(with no charge): 4
·
Number of Neutrons
(most common/stable nuclide): 5
·
Number of Protons: 4
·
Oxidation
States: 2
·
Valence
Electrons: 2s2
Electron
Dot Model

Chemical
Properties of Beryllium
·
Electrochemical
Equivalent: 0.16812g/amp-hr
·
Electron
Work Function: 4.98eV
·
Electronegativity: 1.57
(Pauling); 1.47 (Allrod Rochow)
·
Heat
of Fusion: 12.2kJ/mol
·
Incompatibilities:
acids and strong bases, carbon
tetrachloride, phos- phorous 3-chlorolithium, caustics, chlorinated
hydrocarbons, oxidizers, molten lithium.
·
Ionization Potential
o
First: 9.322
o
Second: 18.211
o
Third: 153.893
·
Valence
Electron Potential (-eV): 82
·
Boiling
Point: 3243K
2970°C
5378°F
·
Coefficient
of lineal thermal expansion/K-1:
0.0000116E-6
·
Conductivity
Electrical: 0.313 106/cm 
Thermal:
2.01 W/cmK
·
Density:
1.848g/cc @ 300K
·
Description:
Strong, hard, gray-white metal.
Lightest rigid metal. Formerly called glucinium (Gl).
·
Elastic Modulus:
o
Bulk: 110/GPa
o
Rigidity: 156/GPa
o
Youngs: 318/GPa
·
Enthalpy of Atomization: 326.4
kJ/mole @ 25°C
·
Enthalpy of Fusion: 11.72
kJ/mole
·
Enthalpy of Vaporization: 294.7
kJ/mole
·
Flammablity Class:
Non-combustible solid (except as dust)
·
Freezing Point: see melting point
·
Hardness Scale
o
Brinell: 600 MN m-2
o
Mohs: 5.5
o
Vickers: 1670 MN
m-2
·
Heat
of Vaporization: 292.4kJ/mol
·
Melting
Point: 1551K
1278°C
2332°F
·
Molar Volume: 4.88 cm3/mole
·
Pysical State
(at 20°C & 1atm): Solid
·
Specific
Heat: 1.82J/gK
·
Vapor
Pressure 4.18kPa
http://environmentalchemistry.com/yogi/periodic/
|

|
The structure of like
H. Atom
I describe on
Basis M-51 galaxy.
Small and large formations must have
The same behavior.
{A. Einstein}
This galaxy
clearly
show two different swirls connected by two energetic
path and is completely independent wave
{Quantum} formation.
=.
|
The structure of like H. Atom
Helium atom
Lithium atom strcture
Beryllium atom structure
See H, He, Li atoms {Tejman}.
Beryllium atom structure
Energetic matter in quantum {atom} must have two
semi-loop, electric and magnetic properties.
|

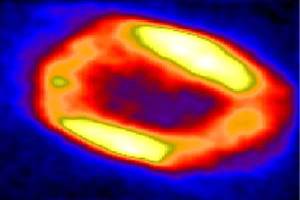
nebula 7027
|

|
Two unclosed quantum{photons} create common
energetic swirl
One unclosed quantum formation {Berylium atom}.
I copy different celestial quantum structures that may
be can help to understand Beryllium atom {quantum} structure and behavior.
For understand
energetic matter behavior we needs a lot of common sense, because
spontaneous, wildest behavior of energetic matter {Heisenberg's Uncertainty Principle and
Schrödinger’s Superposition}.
never create matrix formation only like formations. but
they have the same
vibration frequency because every atom is independent
unclosed quantum formation.
Beryllium atom in
different energetic levels can obtain different structures.
But always guard
of its quantum {atom} formation.
Summery:
Beryllium atom is stabile and is composed by two
a - formations {two Helium formations}
© Copyright:
Dr. Tejman Chaim, Henry August 2007
http://www.grandunifiedtheory.org.il/
The theory of everything
|
![[Bohr Model of Beryllium]](Beryllium_atom_structure_files/image001.gif)