|
Boron-B: atom structure.
Wave theory-United nature theory
Based: of nature and laboratory observations.
Tejman Chaim Henry Dr.
United nature theory-Wave theory
Name:
Boron
Symbol:
B
Atomic Number:
5
Atomic Mass:
10.811 amu
Melting Point:
2300.0 °C (2573.15 K, 4172.0 °F)
Boiling Point:
2550.0 °C (2823.15 K, 4622.0 °F)
Number of
Protons/Electrons: 5
Number of Neutrons:
6
Classification:
Metalloid
Crystal Structure:
Rhombohedral
Density @ 293 K:
2.34 g/cm3
Color:
brownish
|
![[Bohr Model of Boron]](Boron_atom_structureweb_files/image001.gif)
|
|
Number of Energy
Levels: 2
First Energy Level:
2
Second Energy Level:
3
|
|
Isotope |
Half Life |
|
B-10 |
Stable |
|
B-11 |
Stable |
Date of Discovery:
1808
Discoverer:
Sir Humphry Davy, J.L Gay-Lussac
Name Origin:
From borax and carbon
Uses: heat
resistant alloys
Obtained From:
kernite
Taken
from: http://www.chemicalelements.com/elements/b.html
·
Series: Metalloids
(Nonmetal)
Atomic Structure of Boron
·
Atomic
Radius: 1.17Å
·
Atomic
Volume: 4.6cm3/mol
·
Covalent
Radius: 0.82Å
·
Cross
Section (Thermal Neutron Capture) a/barns:
767 a/barns:
767
·
Crystal
Structure: Rhombohedral

·
Electron Configuration:
1s2 2s2p1
·
Electrons per Energy
Level: 2,3
Shell
Model
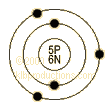
·
Ionic
Radius: 0.23Å
·
Filling
Orbital: 2p1
·
Number of Electrons
(with no charge): 5
·
Number of Neutrons
(most common/stable nuclide): 6
·
Number of Protons: 5
·
Oxidation
States: 3
·
Valence
Electrons: 2s2p1
Electron Dot Model

Chemical
Properties of Boron
·
Electrochemical
Equivalent: 0.1344g/amp-hr
·
Electron
Work Function: 4.45eV
·
Electronegativity: 2.04 (Pauling); 2.01 (Allrod Rochow)
·
Heat
of Fusion: 50.2kJ/mol
·
Incompatibilities:
·
Ionization Potential
o
First: 8.298
o
Second: 25.154
o
Third: 37.93
·
Valence
Electron Potential (-eV): 190
Physical Properties
of Boron
·
Atomic
Mass Average: 10.811
·
Boiling
Point: 4275K
4002°C
7236°F
·
Coefficient
of lineal thermal expansion/K-1: 5E-6
·
Conductivity
Electrical: 1.0E-12 106/cm 
Thermal: 0.274 W/cmK
·
Density: 2.34g/cc @ 300K
·
Description:
Yellow-brown non-metallic crystal.
·
Elastic Modulus:
o
Bulk: 320/GPa
·
Enthalpy of Atomization: 573.2 kJ/mole @ 25°C
·
Enthalpy of Fusion: 22.18 kJ/mole
·
Enthalpy of Vaporization: 480 kJ/mole
·
Flammablity Class:
·
Freezing Point: see melting point
·
Hardness Scale
o
Mohs: 9.3
o
Vickers: 49000 MN m-2
·
Heat
of Vaporization:
489.7kJ/mol
·
Melting
Point: 2573K
2300°C
4172°F
·
Molar Volume: 4.68 cm3/mole
·
Pysical State (at 20°C & 1atm): Solid
·
Specific
Heat: 1.02J/gK
Notes below
Taken from: http://environmentalchemistry.com/
|

|
The
structure of like
H.
Atom
I
describe on
Basis
M-51 galaxy.
Small
and large formations must have
The
same behavior.
{A.
Einstein}
This
galaxy clearly
show
two different swirls connected by two energetic path and is completely
independent wave
{Quantum}
formation.
=. |
The structure of like H. Atom
Helium atom
Lithium atom structure.
Beryllium atom structure
See H, He, Li atoms {Tejman}.
Beryllium atom structure
Boron atom structure
Boron atom structure
Bo Atomic Mass: 10.811
amu
Isotopes
|
Isotope |
Half Life |
|
B-10 |
Stable |
|
B-11 |
Stable |
Pictures
from sky “laboratory” can help understand energetic matter behavior.
That
is not just like that Boron atom formations, but we must understand the
energetic matter behavior
I give
her only foggiest
(idea) of Boron and atoms behavior but that is only beginning of the road
for atomic research.
For understanding the
energetic matter behavior we need a lot of common sense, because every atom
is independent
un-closed quantum
formation.
The spontaneous and the
wildest behavior of energetic matter {Heisenberg's Uncertainty Principle and
Schrödinger’s Superposition} can’t create identical matrix, only similar
formations. and that give to like atoms their specific hue, like specific hue
to every man, but like atoms have the same vibration and wave
formation,
|
M57: The Ring Nebula
Credit: The Electronic Universe
Project, Nelson Caldwell |

NGC 4361: Galaxy Shaped
Planetary Nebula
Credit: Bill Keel, U.
Alabama |
From space laboratory we can learn energetic matter
behavior.
Summary:
Every atom is independent unclosed gravitational
wave {quantum} formation and similar atoms have the same vibration.
©
Copyright: Dr. Tejman Chaim, Henry , August 2007
http://www.grandunifiedtheory.org.il/
The theory of everything
|