|
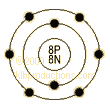
Oxygen-O atom structure
This like “simple” formation” is the most sophisticated
creation.
Wave theory-United nature theory
For understand atomic structure we must
to team up laboratory and nature observations.
Tejman Chaim Henry Dr.
OAtomic
Number:8
·
Group:
16
·
Period:
2
·
Series: Nonmetals
Structure of Oxygen
·
Atomic
Radius: 0.65Å
·
Atomic
Volume: 14cm3/mol
·
Covalent
Radius: 0.73Å
·
Cross
Section (Thermal Neutron Capture)  a/barns: 0.00019 a/barns: 0.00019
·
Crystal
Structure: Cubic
·
Electron Configuration:
1s2 2s2p4
·
·
Electrons per Energy
Level: 2,6
Shell
Model
·
Ionic
Radius: 1.4Å
·
Filling
Orbital: 2p4
·
Number of Electrons
(with no charge): 8
·
Number of Neutrons
(most common/stable nuclide): 8
·
Number of Protons: 8
·
Oxidation
States: -2,-1
·
Valence
Electrons: 2s2p4
Electron
Dot Model

Chemical Properties of Oxygen
·
Electrochemical
Equivalent: 0.29847g/amp-hr
·
Electron
Work Function:
·
Electronegativity: 3.44 (Pauling); 3.5 (Allrod Rochow)
·
Heat
of Fusion: 0.22259kJ/mol
·
Incompatibilities:
oxidizable materials
·
Ionization Potential
o
First: 13.618
o
Second: 35.117
o
Third: 54.934
·
Valence
Electron Potential (-eV): -20.6
Physical Properties of
Oxygen
·
Atomic
Mass Average: 15.9994
·
Boiling
Point: 90.33K
-182.82°C
-297.08°F
·
Coefficient
of lineal thermal expansion/K-1: N/A
·
Conductivity
Electrical:
Thermal: 0.0002674 W/cmK
·
Density: 1.429g/L @ 273K & 1atm
·
Description:
Colorless, odorless,
tasteless gas
·
Enthalpy of Atomization: 249.4 kJ/mole @ 25°C
·
Enthalpy of Fusion: 0.22 kJ/mole
·
Enthalpy of Vaporization: 3.41 kJ/mole
·
Flammablity Class: Non-flammable gas (Oxidizer)
·
Freezing Point: see melting point
·
Heat
of Vaporization: 3.4099kJ/mol
·
Melting
Point: 50.5K
-222.65°C
-368.77°F
·
Molar Volume: 14 cm3/mole
·
Optical Refractive Index: 1.000271 (gas) 1.221 (liquid)
·
Pysical State (at 20°C & 1atm): Gas
·
Specific
Heat: 0.92J/gK
o
Discoverer: Joseph Priestley, Carl Wilhelm Scheele
o
Discovery Location: Leeds England (Priestley)/Uppsala Sweden (Scheele)
o
Discovery Year: 1774
o
Name Origin:
Greek: oxus (acid) and
gennan (generate).
§
Atmosphere/p.p.m.: 209500
§
Sun
(Relative to H=1E12):
N/A
o
Sources of Oxygen:
Obtained primarily
from by liquification and then fractional distillation of the air. Annual
world wide production is around 100,000,000 tons.
o
Uses of Oxygen:
Forms almost 21% of
atmosphere. Used in steel making, welding, water purification, cement. It is
also required for supporting life and combustion.
o
Additional Notes:
Oxygen is the most
abundant element on the surface of the earth.
http://environmentalchemistry.com/yogi/periodic/N.html
l
|

|
The structure of like
H. Atom
I describe on
Basis M-51 galaxy.
Small and large formations must have
The same behavior.
{A. Einstein}
This galaxy
clearly
show two different swirls connected by two energetic
path and is completely independent wave
{Quantum} formation.
|
The structure of like H. Atom
Helium atom
Lithium atom structure
Beryllium atom structure
See H, He, Li atoms {Tejman}.
Beryllium atom structure
Boron atom structure
Boron atom structure
Carbon atom structure
Carbon atom structure
Nitrogen atom
Oxygen atom
Every atom-quantum is composed by two perpendicular
sem-loops: electric and magnetic.
Summary:
Every atom-quantum-wave formation {bubble -10
dimensions} is composed by two swirls {electric and magnetic} connected by
energetic paths.
This like “simple” formation” is the most sophisticated
creation.
© Copyright: Dr.
Tejman Chaim, Henry August 2007
http://www.grandunifiedtheory.org.il/
The theory of everything
|